Inequalities in cancer incidence in Wales by socio-demographic characteristics, 2011-2020
Published: Thursday 5th June 2025
This report presents an analysis of individual levels of deprivation and socio-demographic characteristics, including ethnicity and overcrowding. For the first time, we have linked cancer registry data to data from the 2011 Census in England and Wales. This gives us a new, granular snapshot of the socio-demographic profile of people diagnosed with cancer in Wales. This is vital in reducing inequalities and inequities, and in planning prevention and services for future generations in Wales. Following the work in this report, further work is recommended to fully understand the impact of these socio-demographic characteristics on cancer incidence, in addition to other possible factors.
Our annual official cancer statistics are usually only able to present inequalities for people in Wales’ characteristics based on age and biological sex. Routinely, for various measures of deprivation, we can only assign people to a level of socio-economic deprivation based on the area in which they live rather than looking at individual characteristics. This may not necessarily reflect their personal level of inequity or deprivation, meaning area-level analysis of cancer incidence deprivation can lose detail. The linkage in this analysis has enabled us to look at individual or household measures of inequality.
Headlines
- There was a higher proportion of prostate cancer in the Black population and a higher proportion of female breast cancer in the Asian population. The White population had the highest cancer incidence rate overall. For stage at diagnosis, the Asian population had the highest proportion of Stage 1 (early) diagnoses whilst the Mixed ethnicity population had the highest proportion of Stage 4 (late) diagnoses.
- People living in overcrowded accommodation were found to have a cancer incidence rate seven times higher than those living with two or more spare rooms, after adjusting for age.
- After accounting for age, those who live in social housing had a cancer incidence rate nearly three times higher than those who own their home outright.
- The highest rates of cancer were seen in people who worked as process, plant and machine operatives in both retirees and those in active occupation. This occupation also had a higher proportion of late-stage diagnoses compared to some other occupations.
- It is likely that income and other forms of individual-level socioeconomic deprivation are playing a part in the difference between the cancer incidence rates by housing overcrowding, tenure and occupation above. This highlights the need for further investigation into how this deprivation gap can be narrowed.
Sections
1. Overview
International studies suggest cancer incidence rates may vary by the individual demographic characteristics of people (Denny et al., 2019), as well as by area-based measures of deprivation. Our report presents the results of an investigation into inequalities and inequities amongst people with cancer in Wales with different individual socio-demographic characteristics including ethnicity, occupation, household tenure, and overcrowding in households.
It is important to investigate characteristics at the individual level that could contribute to inequalities in cancer incidence between people with cancer in Wales. This gives an opportunity to understand health inequities in society, and to inform policies that may in turn reduce them. Nearly four in ten cancer cases in the UK in 2015 could be attributed to known risk factors (Brown et al., 2018). Better understanding of risk factors and inequities in cancer incidence can assist in targeting cancer prevention and other public health initiatives. In addition, increased knowledge of the pattern of potential inequalities could help the planning of primary and secondary health care services. Improved services and interventions could, therefore, lead to a reduction in the incidence of preventable cancers, and better outcomes for people with cancer in Wales.
This work was carried out by the Welsh Cancer Intelligence and Surveillance Unit (WCISU) within Public Health Wales. We used the Secure Anonymised Information Linkage (SAIL) Databank based in Swansea University to anonymously and confidentially link quality-assured, population-based cancer registry incidence and 2011 Census data. This work analysed ‘all cancers combined excluding non-melanoma skin cancer (NMSC)’ and four main cancer types: female breast, colorectal (bowel), prostate and lung. ‘Other cancer types’ refers to all cancer types excluding those reported separately (i.e. female breast, lung, prostate, and colorectal cancers) and NMSC. The people whose data was included in this analysis were diagnosed between 2011 and 2020, inclusive.
We present results of cancer incidence by proportion (%), crude rate and European age-standardised rates. Further information on the data and methods used in this analysis, including their limitations, can be found in the accompanying technical guide.
2. Cancer incidence by ethnicity
Self-reported ethnicity in the 2011 Census (Office for National Statistics, 2011) and the SAIL ethnicity spine (Akbari, Torabi, Bedston et al., 2024) were used to identify six broad ethnic groups for analysis:
- Asian/Asian British
- Black/African/Caribbean/Black British
- White English/Welsh/Scottish/Northern Irish/British
- Mixed/Multiple ethnic groups
- Other ethnic groups
- Unknown/unmatched ethnicity.
Note: The ethnic groups analysed in this publication will be referred to as ‘Asian,’ ‘Black,’ ‘White,’ ‘Mixed ethnicity,’ and ‘Other ethnicity’ for succinctness.
Recording of ethnicity in health data is often incomplete. Therefore, the use of Census data was vital to robustly identify ethnicity and gain insight into potential inequalities in cancer incidence between ethnic groups in Wales. Cancer Research UK (2022) suggests that differences in cancer incidence between ethnic groups, for certain cancer types, are likely driven by non-genetic cancer risk factors such as smoking rates, and overweight and obesity rates. However, wider determinants of health such as the social, economic and physical environment, and a person’s individual characteristics also play a major role. Evidence suggests that minority ethnic groups may experience higher rates of deprivation in England (Ministry of Housing, Communities and Local Government, 2020). As minority ethnic groups become more established, their risk factor prevalence, and subsequent cancer incidence, has moved increasingly towards that of the population as a whole (Delon et al., 2022).
Ethnicity and age in Wales
Age is the biggest risk factor for cancer, with the majority of cancers occurring in older people. When we look at the proportion of cancers occurring by ethnicity for all ages (Table 1), the White ethnic group has substantially higher numbers as it has a larger population at risk of cancer overall compared to other ethnic groups in Wales. It also has the largest proportion of older people. However, inequalities may still exist between ethnic groups, and this could be masked by the older, larger White population. This is overcome by analysing the age-standardised incidence rates for each ethnic group (see Figure 1).
Table 1: Counts and proportion (%) of cancer incidence of all cancers combined excluding NMSC by ethnicity for persons of all ages in Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office of National Statistics 2011 Census; SAIL Databank.
| Ethnicity | Men | Women | Persons |
|---|---|---|---|
| White | 97,250 (96.4%) | 89,476 (96.4%) | 186,726 (96.4%) |
| Unknown/Unmatched | 2,213 (2.2%) | 1,968 (2.1%) | 4,181 (2.2%) |
| Asian | 628 (0.6%) | 692 (0.8%) | 1,320 (0.7%) |
| Mixed ethnicity | 365 (0.4%) | 329 (0.4%) | 694 (0.4%) |
| Black | 263 (0.3%) | 170 (0.2%) | 433 (0.2%) |
| Other ethnicity | 194 (0.2%) | 156 (0.2%) | 350 (0.2%) |
It is important to look at the age breakdowns of different ethnic group populations within Wales (Table 2). This helps us to determine which ethnicities have a larger number of people at higher risk of cancer due to older age and reflects the different age structure of ethnic group populations in Wales (see Appendix: Figures 15-19 for population breakdowns by ethnic group).
Table 2: Cancer incidence by counts and proportion (%) of cases by age and ethnicity for all cancers combined excluding NMSC, persons, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit (WCISU) cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
| Age group | Asian | Black | Mixed ethnicity | Other ethnicity | White | Unknown/Unmatched |
|---|---|---|---|---|---|---|
| Less than 35 | 145 (11%) | 29 (7%) | 49 (7%) | 31 (9%) | 4,171 (2%) | 73 (2%) |
| 35-49 | 248 (19%) | 64 (15%) | 105 (15%) | 74 (21%) | 11,434 (6%) | 235 (6%) |
| 50-64 | 422 (32%) | 151 (35%) | 185 (27%) | 121 (35%) | 44,665 (24%) | 904 (22%) |
| 65+ | 505 (38%) | 189 (44%) | 355 (51%) | 124 (35%) | 126,456 (68%) | 2,969 (71%) |
Incidence by ethnic group, cancer type and stage
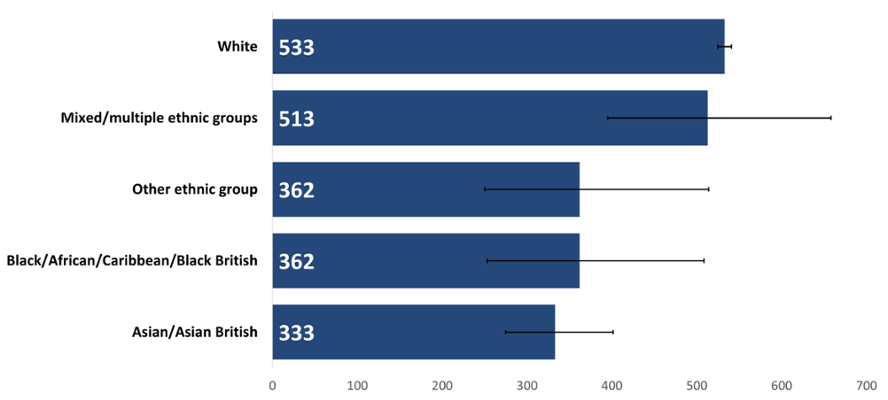
The White ethnic group had the highest age-standardised rate of cancer incidence in Wales in 2020 (Figure 1); 1.6 times higher than the lowest rate which was in the Asian group, and 1.5 times higher than the rates for the Black and Other ethnicity groups. The Mixed ethnic group’s rate was not statistically different to the White group. A study in England (Delon et al., 2022) also found that incidence rates for most cancer types were lower in non-White minority ethnic groups compared to the White ethnic group. It is unclear why rates were highest in the White population once accounting for population ages in each ethnic group. More work is needed to investigate factors that could be affecting this such as deprivation, genetics, or prevalence of risk factors such as smoking and obesity.
Figure 1: Incidence by ethnicity of all cancers combined excluding NMSC, European age-standardised rate per 100,000, persons, all ages, Wales, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
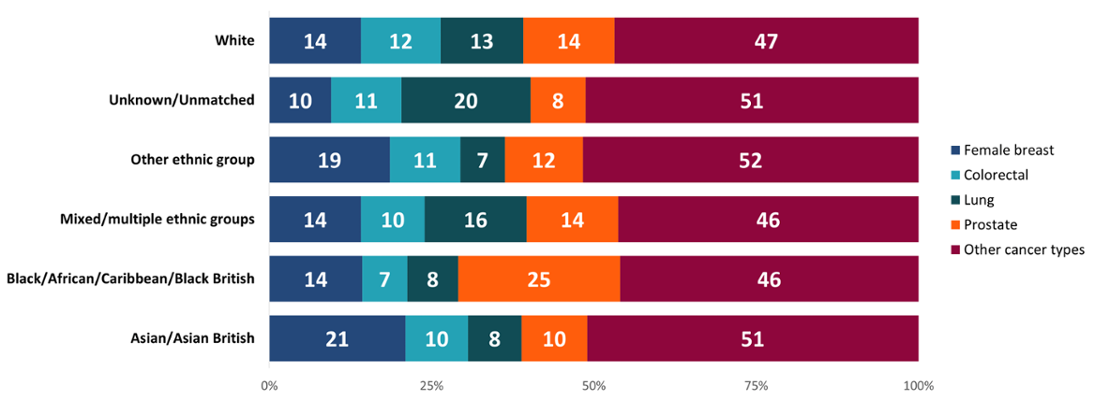
Differences can be seen between the types of cancers diagnosed in each ethnic group in Wales (Figure 2). For instance, the Asian group had a higher proportion of female breast cancer diagnoses compared to other cancer types, whilst the Black group had a higher proportion of prostate cancer diagnoses within their ethnic group. This supports evidence that Black men are at higher risk of developing prostate cancer (Prostate Cancer UK, 2023). The White group had a more even spread of cancer diagnoses across the different types. This may be in part as they form most of the population in Wales, especially at older ages, so had more diagnoses overall. If the age profile of all ethnic groups was the same, we may see an increase in cancers which affect mainly older people, in turn reducing the dominance of some cancer sites within ethnic minority groups in Wales.
Figure 2: Proportion (%) of each cancer type within each ethnic group, persons, all ages, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
The stage of a cancer describes tumour size and how far it has spread. An earlier stage at diagnosis is associated with improved outcomes. The Mixed ethnicity group had a higher proportion of late-stage (Stage 3 and 4) diagnoses, whilst the Asian and Other ethnicity groups had higher proportions of early-stage (Stage 1 and 2) diagnoses (Figure 3). The Black group had a higher proportion of Stage 2 diagnoses compared to other stages. These results may indicate variation in types of cancers diagnosed in people of different ethnicities, differences in health-seeking behaviour or access to health services and experiences along the care pathway.
Figure 3: Proportion (%) of each stage at diagnosis by ethnic group, all cancers combined excluding NMSC, persons, all ages, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
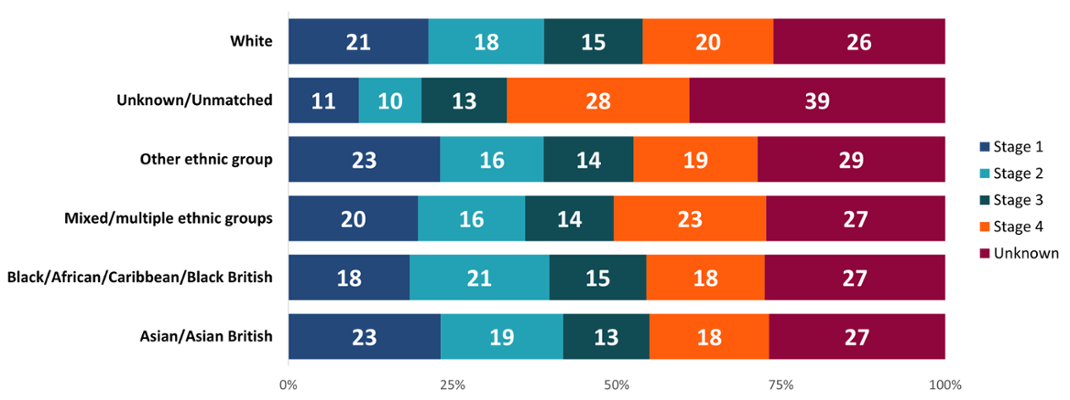
The Asian ethnic group had a higher proportion of female breast cancer diagnoses compared to other cancer types diagnosed in this group (Figure 3). However, studies have shown that barriers exist in the UK for breast and cervical screening uptake amongst Black, Asian and Minority Ethnic women. This includes health service delivery, language barriers, and gaps in awareness or support (Bolarinwa and Holt, 2023). While some of these studies look at ethnic groups separately, others group them into just one combined minority ethnic group. Previous work in Wales has also identified lower bowel screening uptake among ethnic minority groups (Bright et al., 2022). Further work to investigate and address any barriers in Wales could help to improve outcomes for cancer patients.
3. Cancer incidence by overcrowding
In this report, overcrowding is based on the ‘number of persons per bedroom in household’ indicator in the Office for National Statistics 2011 Census. Those living in communal establishments such as care homes were excluded. The overcrowding indicator may indicate levels of deprivation. Those living with fewer bedrooms than required are more likely to be deprived. Deprivation is a risk factor for cancer in part because it is linked to higher prevalence of smoking, obesity, and alcohol consumption. Deprivation also contributes to barriers to accessing healthcare or seeking help, and experiences of longer wait times for care (Cancer Research UK, 2025).
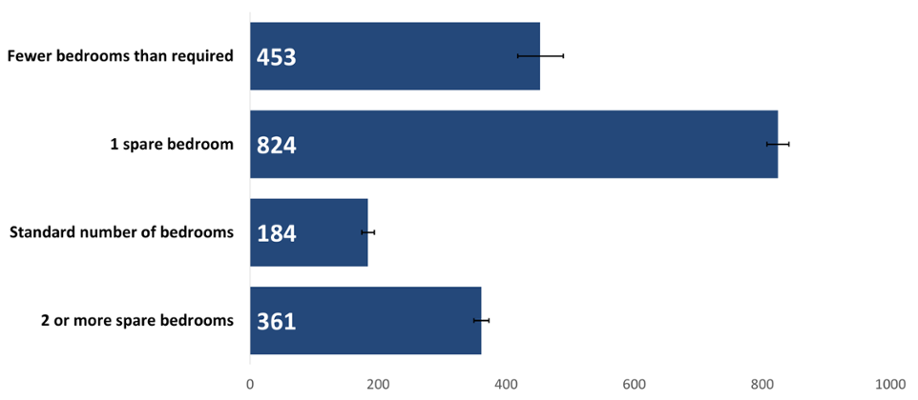
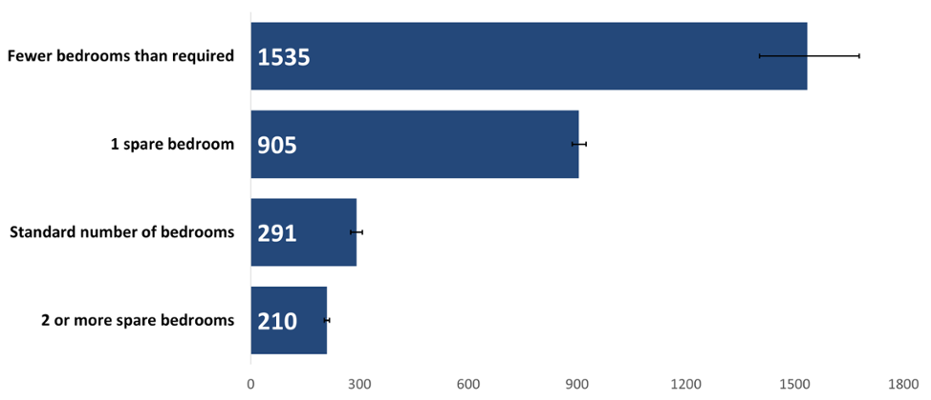
The crude rate of cancer incidence by number of bedrooms was significantly higher in people with one spare bedroom followed by those with fewer bedrooms than required (Figure 4). This could reflect the role of both age and deprivation as risk factors for cancer. When we account for the age structure of people with one spare bedroom, Figure 5 shows us that the relationship between having a spare bedroom and age is only part of the picture, with deprivation having a strong impact on cancer incidence rates. On the other hand, people living in housing with fewer bedrooms than needed may be more deprived.
Figure 4: Incidence of all cancers combined excluding NMSC by number of bedrooms, crude rate per 100,000, persons, all ages, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
The age-standardised rate of cancer incidence was 70 per cent higher for those with fewer bedrooms than required compared to the next highest rate in people with one spare bedroom (Figure 5). However, we can see that differing types of deprivation may be affecting cancer incidence in terms of overcrowding. If no factors other than overcrowding (as an indicator of individual-level deprivation) were at play, we would expect to see people with a standard number of bedrooms having the next highest rate. Instead, people with one spare bedroom had the second highest rate of cancer diagnoses. This could be because people living in housing with fewer bedrooms than required may be more likely to be experiencing material or financial deprivation.
In contrast, those with spare bedrooms may be experiencing more social deprivation such as isolation, resulting in the increased rates we see in both groups. Older people may be impacted more by loneliness and social isolation which can affect health and is associated with an increased risk of mortality (Agni Nakou et al., 2025). We may see more of this in Wales as the population becomes more elderly. These factors, and others which may affect the health of the Welsh population, would benefit from further exploration.
Figure 5: Incidence of all cancers combined excluding NMSC by number of bedrooms, European age-standardised rate per 100,000, persons, all ages, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
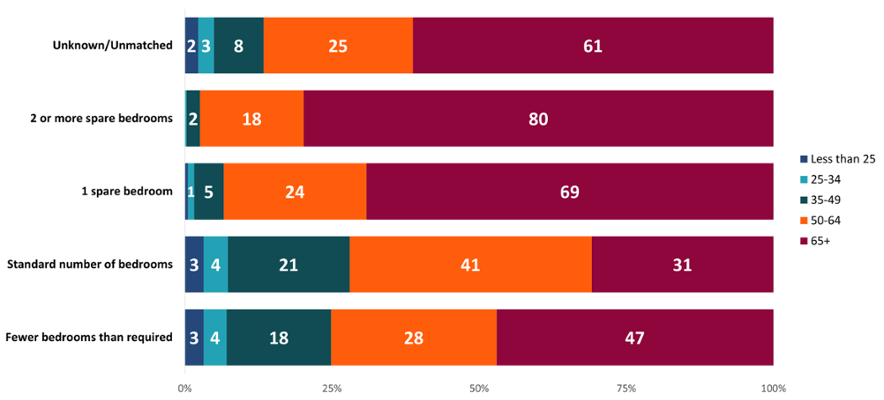
The ’one spare bedroom’ and ’two or more spare bedrooms’ categories had higher proportions of people over 65 years of age (Figure 6). However, we can see that a large proportion of people with cancer diagnosed between 2011 and 2020 who had fewer bedrooms than required were also 65 years of age or over. This reflects the impact of age as a major risk factor for cancer and the aging population in Wales. This is also evidence that factors other than age are contributing to a higher rate of cancer incidence in people with one spare bedroom. Rates were higher in this category (Figure 4 & Figure 5) despite having a smaller proportion of people at older ages compared to those with two or more spare bedrooms.
Figure 6: Proportion (%) of age at diagnosis for incidence counts of all cancers combined excluding NMSC by number of bedrooms, persons, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
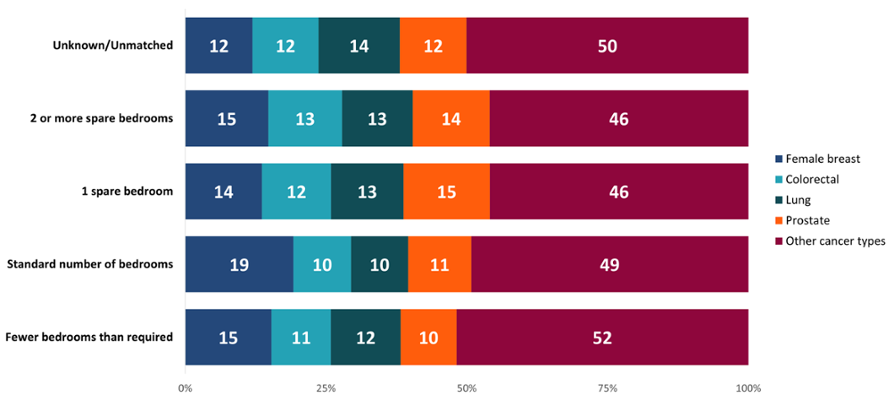
Looking at the most common cancer types, there was a higher proportion of female breast cancer diagnoses in people with a standard number of bedrooms compared to the other groups (Figure 7). This may contribute to the different pattern seen in Figure 6 for those living with a standard number of bedrooms. Women are called for breast screening from age 50 in Wales and female breast cancer rates start to increase at a younger age than for the other cancer types examined, possibly contributing to the different pattern seen here.
Figure 7: Proportion (%) of cancer types by number of bedrooms, persons, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
4. Cancer incidence by household tenure
Household tenure is a classification of whether a house is rented or owned. Six categories were analysed and were grouped together for some of the charts:
- privately rented
- living rent free
- socially rented
- shared ownership (part owned/part rented)
- owned with a mortgage or loan
- owned outright.
Those living in communal establishments such as care homes were excluded. Social rented housing refers to rented housing provided by local councils or housing associations, with applicants given priority if they are homeless, living in overcrowded circumstances or have a medical need. This characteristic may indicate levels of deprivation, with those owning their own property being less likely to be deprived compared to those who do not own their home. Lower quality housing is a major driver of health inequalities and poverty in the UK and can majorly impact people’s health and well-being (Faculty of Public Health, 2024).
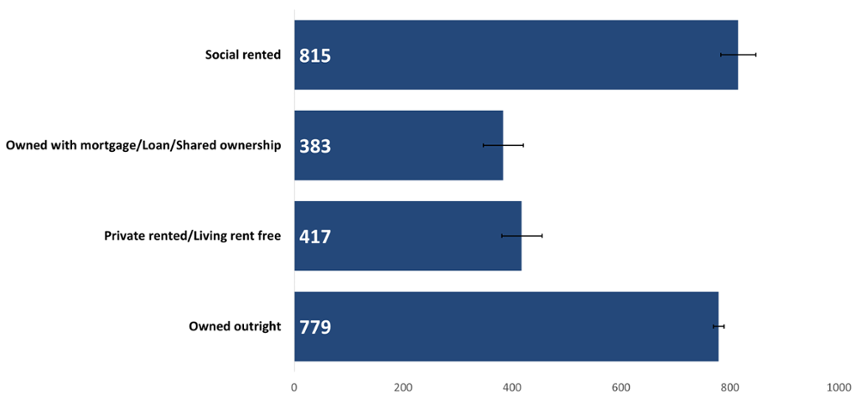
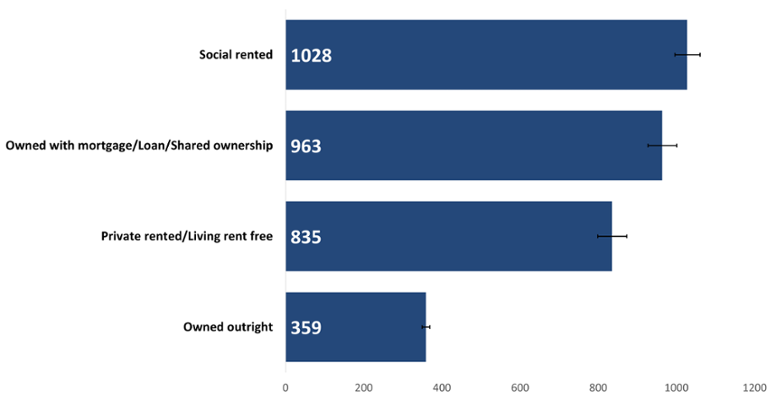
The highest cancer incidence crude rates occurred in both those living in social rented housing and those owning their property outright (Figure 8). As people who own their home outright are likely to be older and less deprived, this displays the influence of older age on cancer incidence. On the other hand, those living in social housing may be more likely to be experiencing deprivation in some way, conversely reflecting the influence of deprivation on cancer incidence.
Figure 8: Incidence of all cancers combined excluding NMSC by household tenure, crude rate per 100,000, persons, all ages, Wales, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
The age-standardised cancer incidence rate for those living in social rented housing was 3 times higher than for those who owned their home outright (Figure 9). The lowest rate was seen in those who owned their home outright. However, there was not a significant difference in rate between social rented housing and those who own their home with a mortgage/loan/shared ownership.
Figure 9: Incidence of all cancers combined excluding NMSC by household tenure, European age-standardised rate per 100,000, persons, all ages, Wales, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
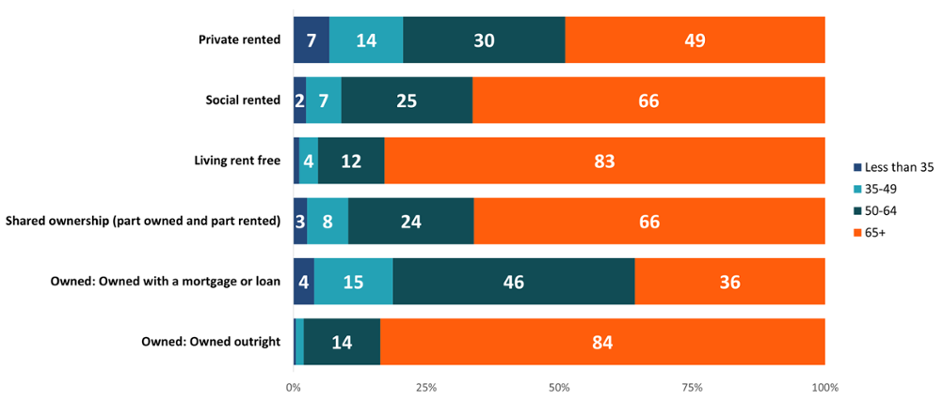
A large majority of those owning their home outright or living rent free were over 65 years of age (Figure 10). There was a high proportion of cancer in those aged 50-64 who owned their home with a mortgage or loan. Similarly to the pattern seen when looking at number of bedrooms (Section 3), there was a higher proportion of female breast cancer in people aged 50-64 who owned their home with a mortgage compared to other types of household tenure. According to the Office for National Statistics (2020), home ownership amongst older people increased in recent years, with an increasing number also having paid off their mortgage by the age of 65 in England. However, younger generations are now less likely to own their own home, which may have implications for their future health and well-being as more people will continue to live in social or private-rented housing (ONS, 2020).
Figure 10: Proportion (%) of ages for all cancers combined excluding NMSC by household tenure, persons, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
5. Cancer incidence by occupation
For occupation, two groups of people were analysed: those retired from a former occupation and those economically active at the time of the 2011 Census (ONS), excluding students.
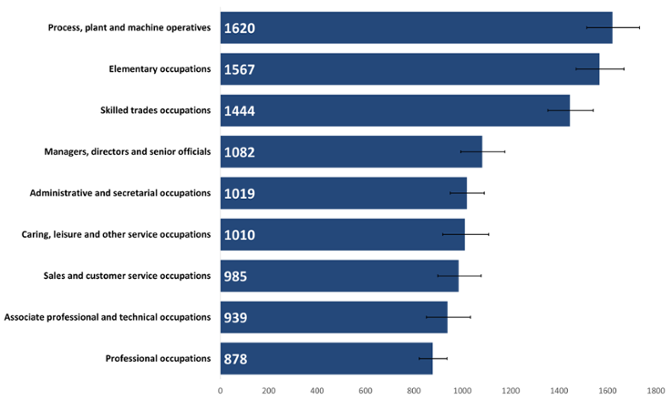
Cancer incidence was significantly higher in retired people whose previous roles were within machine operation, skilled trades or elementary jobs, such as factory workers, tradespeople, retail workers, cleaners and labourers, compared to other occupational groups (Figure 11). This pattern is likely driven by higher cancer incidence rates usually occurring in men in Wales (Public Health Wales, 2024), along with any age-structure differences between the retired occupational groups.
Rates were lowest in technical and professional occupations. This may be due to differing prevalence of risk factors between people in different occupations, such as smoking prevalence or occupational hazards. Some studies have found a link between income, education level, place of residence or occupation and access to screening and treatment as well as levels of health literacy (Li et al., 2024). All of these factors may be at play in these occupational groups.
Figure 11: Incidence of all cancers combined, excluding NMSC, by retired occupation, crude rate per 100,000, persons aged 16+ years, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
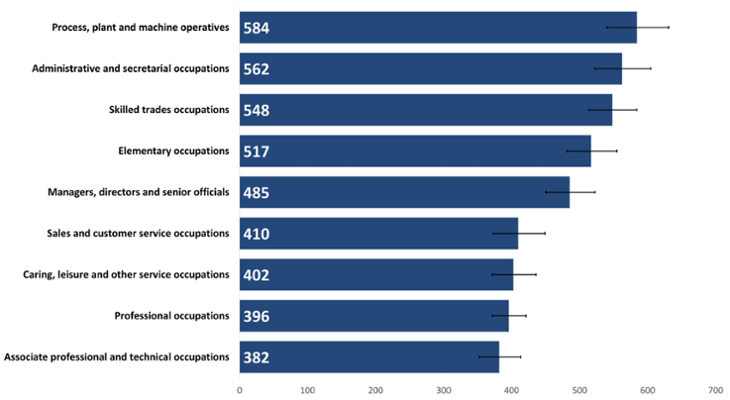
There was less difference between the highest and lowest rates of cancer incidence in people in active occupation (Figure 12) compared to in retirees. The highest cancer incidence rate for those in active occupation was also smaller than the lowest rate seen for retirees, again reflecting the impact of age on cancer incidence.
A different pattern is seen in people in active occupations compared to retirees (Figure 12), with administrative roles now having the second highest rate. There were more than 3 times as many women than men in this occupation group, so this rate is likely to be driven by female breast cancer. Female breast cancer is one of the most common cancers diagnosed in Wales and rates of female breast cancer begin to rise before retirement age (Public Health Wales, 2024).
Figure 12: Incidence of all cancers combined excluding NMSC by active occupation, crude rate per 100,000, persons aged 16+ years, 2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
Female breast cancer was often diagnosed in female-dominated jobs and prostate cancer diagnosed in more male-dominated jobs when looking at active occupation groups (Figure 13). This correlates with the rates seen in Figure 12 for some of these occupations, such as skilled trades and administrative roles. The ’Adult smoking habits in the UK: 2023’ report (ONS, 2024) showed that smoking prevalence was significantly lower in those working in managerial and professional occupations and significantly higher in those working in routine and manual occupations. Whilst the categories used in that report do not directly match with ours, we can see that smoking prevalence may play a part in the lower lung cancer rates we find in professional occupations compared to process, plant and machine operatives or elementary occupations such as factory workers or labourers.
Figure 13: Proportion (%) of each cancer type by active occupation, persons aged 16+ years, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank.
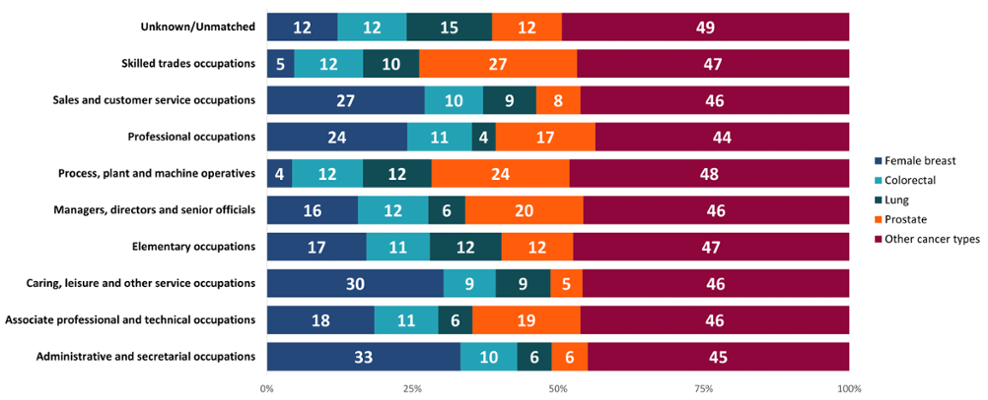
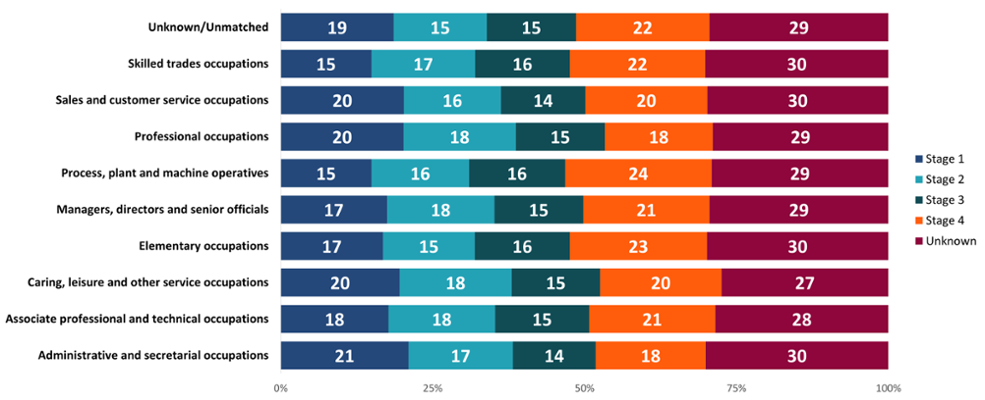
In retirees, some of the male-dominated industries, particularly process, plant and machine operatives, had higher proportions of people diagnosed at a later stage (Figure 14). Meanwhile, some more female-dominated roles such as administrative and secretarial occupations had higher proportions of people diagnosed at an early stage, which may again reflect high female breast cancer diagnoses in older women. This could also be because of different behaviours between the sexes, with men more likely to delay seeking healthcare services compared to women (Banks, 2001). Further work would be needed to examine other possible reasons.
Those in professional occupations had a higher proportion of early-stage diagnoses compared to those working in elementary occupations, such as cleaners or labourers, who had a higher proportion of late-stage diagnoses. This may be due to a difference in the types of cancers diagnosed between these groups, as we saw for those in active occupation (Figure 13). However, it may also suggest potential barriers to healthcare access or differences in health-seeking behaviours. Other research suggests that people living in more deprived areas or with the lowest educational attainment may experience more barriers to healthcare and present later with cancer symptoms (McCutchan et al., 2016). These researchers suggest that this could be due to factors such as poorer awareness of cancer symptoms, various barriers to accessing or attending appointments or fearful beliefs about cancer.
Figure 14: Proportion (%) of cancer diagnoses by early and late stage diagnoses, retired occupation, all cancers combined excluding NMSC, persons aged 16+ years, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; SAIL Databank. See Appendix: Figure 20 for a more granular breakdown of stage at diagnosis for retirees.
6. Conclusion
Thanks to the ability to link high quality whole-population cancer registry data and 2011 Census data through the SAIL databank, we have been able to carry out analysis that is not possible with our cancer registry data alone. This linkage has enabled us to begin to explore the effects of different socio-demographic characteristics on cancer incidence at an individual level rather than our usual area-based deprivation analyses.
While we have been able to provide a snapshot of where differences and potential inequalities are found within these indicators, further work is required to understand the true effect of ethnicity, overcrowding, occupation and other factors on cancer incidence in Wales. The analysis in this report was limited in some areas due to the small numbers of patients in some groupings and the availability of data. Further details on the analysis can be found in the Technical Guide. However, this work provides a strong baseline for further planned analysis on inequalities and cancer incidence and highlights further questions for exploration.
7. Useful links
8. Data files
Download the data in this article - Excel
9. Acknowledgements
This work uses data provided by patients and collected by the NHS as part of their care and support. We also want to acknowledge all data providers who make anonymised data available for research.
We wish to acknowledge the collaborative partnership that enabled the acquisition and access to the de-identified data which led to this output. The collaboration was led by the Swansea University Population Data Science group, under the direction of the Welsh Government Technical Advisory Cell (TAC) and includes the following groups and organisations: the SAIL Databank, Health Data Research UK (HDR UK), Administrative Data Research (ADR) Wales, Digital Health and Care Wales (DHCW, formerly NHS Wales Informatics Service (NWIS)), Public Health Wales, NHS Shared Services and the Welsh Ambulance Services University NHS Trust (WAST). All research has been completed under the permission and approval of the SAIL independent Information Governance Review Panel (IGRP) project number 0911.
We would also like to thank the members of the DATA-CAN-CCC research group for their support and input into this research project.
10. Funding
This study makes use of anonymised data held in the SAIL Databank. We would like to acknowledge all the data providers who make anonymised data available for research.
This work was supported by the Con-COV team funded by the Medical Research Council (grant number: MR/V028367/1).
This work was supported by Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-9006) funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation (BHF) and the Wellcome Trust.
This work was supported by the ADR Wales programme of work. ADR Wales, part of the ADR UK investment, unites research expertise from Swansea University Medical School and WISERD (Wales Institute of Social and Economic Research and Data) at Cardiff University with analysts from Welsh Government. ADR UK is funded by the Economic and Social Research Council (ESRC), part of UK Research and Innovation. This research was supported by ESRC funding, including Administrative Data Research Wales (ES/W012227/1).
This work was supported by the Wales COVID-19 Evidence Centre, funded by Health and Care Research Wales.
11. Appendix
Figures 15-19: Population breakdowns for each ethnic group analysed by sex and age group in Wales, 2021. Welsh Cancer Intelligence and Surveillance Unit; Office for National Statistics Census 2021.
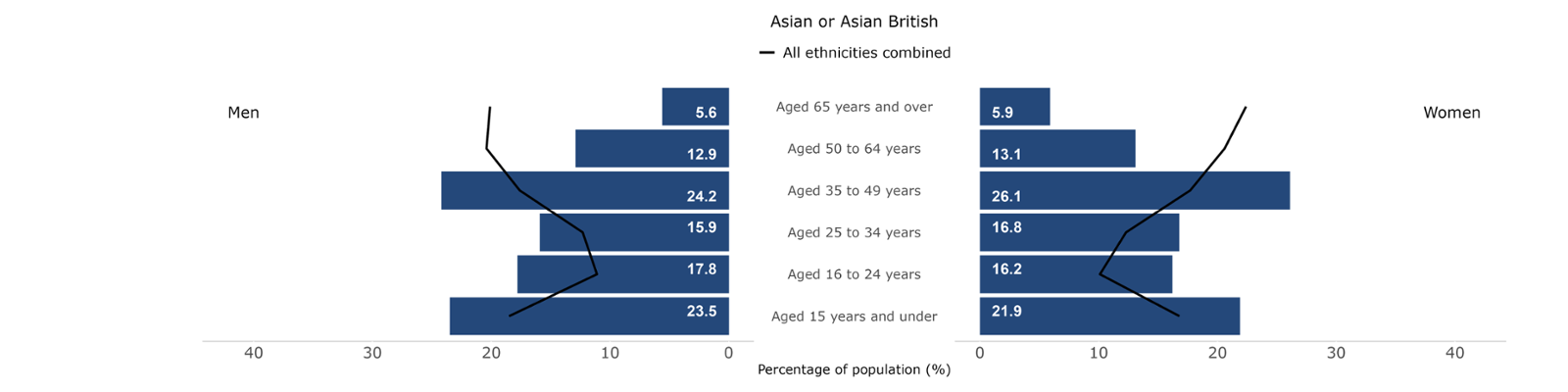
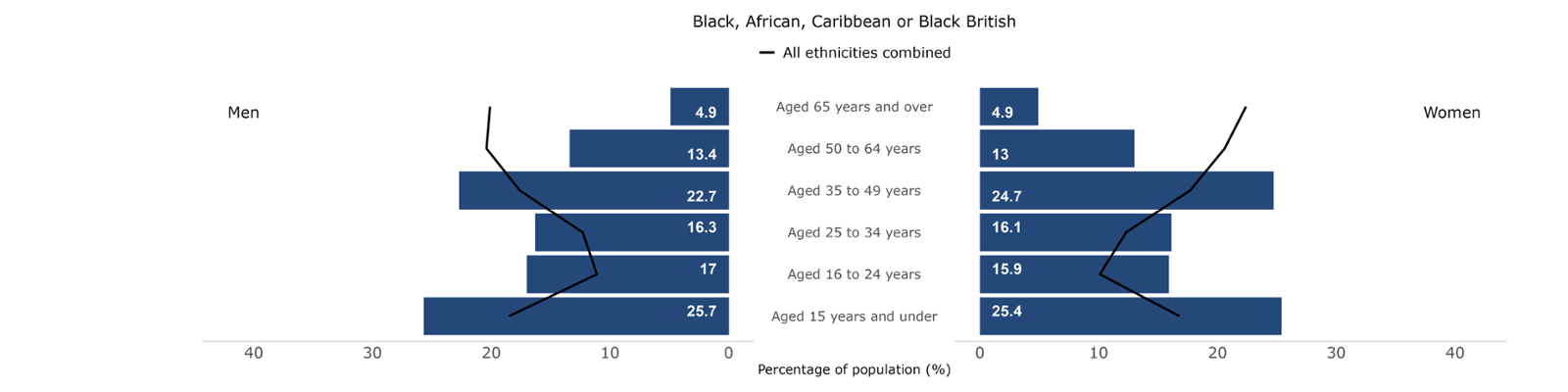
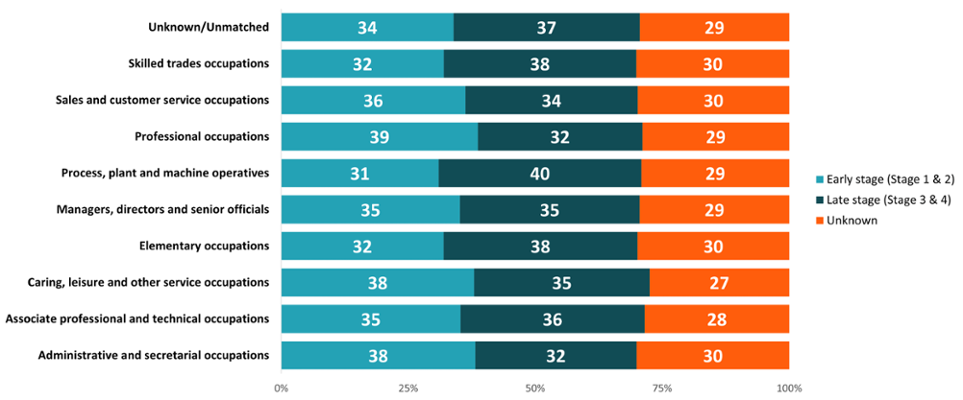
Figure 20: Proportion (%) of cancer incidence by stage at diagnosis, retired occupation, all cancers combined excluding NMSC, persons aged 16+ years, Wales, 2011-2020. Welsh Cancer Intelligence and Surveillance Unit cancer registration data; Office for National Statistics 2011 Census; Office for National Statistics Census 2021; SAIL Databank.
12. References
- Denny, L., Jemal, A., Schubauer-Berigan, M., Islami, F., Vilahur, N., Fidler, M., Sarfati, D., Soerjomataram, I., Catherine de Martel and Vaccarella, S. (2019). Social inequalities in cancer risk factors and health-care access. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK566202/.
- Brown, K.F., Rumgay, H., Dunlop, C., Ryan, M., Quartly, F., Cox, A., Deas, A., Elliss-Brookes, L., Gavin, A., Hounsome, L., Huws, D., Ormiston-Smith, N., Shelton, J., White, C. and Parkin, D.M. (2018). The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. British Journal of Cancer, [online] 118(8), pp.1130–1141. doi:https://doi.org/10.1038/s41416-018-0029-6.
- Office for National Statistics (2011). 2011 Census - Office for National Statistics. [online] Ons.gov.uk. Available at: https://www.ons.gov.uk/Census/2011Census.
- Akbari A, Torabi F, Bedston S, et al. Exploring ethnicity dynamics in Wales: a longitudinal population-scale linked data study and development of a harmonised ethnicity spine. BMJ Open 2024. Available at: 14:e077675. doi: 10.1136/bmjopen-2023-077675
- Cancer Research UK (2022). First data in a decade highlights ethnic disparities in cancer. [online] Cancer Research UK - Cancer News. Available at: https://news.cancerresearchuk.org/2022/03/02/first-data-in-a-decade-highlights-ethnic-disparities-in-cancer/.
- Ministry of Housing, Communities and Local Government (2020). People living in deprived neighbourhoods. [online] gov.uk. Available at: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/people-living-in-deprived-neighbourhoods/latest/#title
- Delon, C., Brown, K.F., Payne, N.W.S., Kotrotsios, Y., Vernon, S. and Shelton, J. (2022). Differences in Cancer Incidence by Broad Ethnic Group in England, 2013–2017. British Journal of Cancer, [online] 126(12). doi:https://doi.org/10.1038/s41416-022-01718-5.
- Prostate Cancer UK (2023). Black men and prostate cancer. [online] Prostate Cancer UK. Available at: https://prostatecanceruk.org/prostate-information-and-support/risk-and-symptoms/black-men-and-prostate-cancer.
- Bolarinwa, O.A. and Holt, N. (2023). Barriers to breast and cervical cancer screening uptake among Black, Asian, and Minority Ethnic women in the United Kingdom: evidence from a mixed-methods systematic review. BMC health services research, [online] 23(1), p.390. doi:https://doi.org/10.1186/s12913-023-09410-x.
- Bright, D., Song, J., Hillier, S., Huws, D.W., Greene, G., Hodgson, K., Akbari, A., Griffiths, R., Davies, A.R. and Gjini, A. (2022). Impact of the temporary suspension of the Bowel Screening Wales programme on inequalities during the COVID-19 pandemic: a retrospective register-based study. Lancet (London, England), [online] 400, p.S25. doi:https://doi.org/10.1016/S0140-6736(22)02235-8.
- Cancer Research UK (2025). Cancer in the UK 2025: Socioeconomic deprivation. [online] Cancer Research UK. Available at: https://www.cancerresearchuk.org/sites/default/files/cancer_in_the_uk_2025_socioeconomic_deprivation.pdf?_gl=1*syv9oo*_gcl_au*OTUwMDgwMTU2LjE3MzY3ODQ5ODA.*_ga*MTU4NDc2MTM0Ny4xNzA4NTIyOTY4*_ga_58736Z2GNN*MTc0MDEzNTkzNi40My4wLjE3NDAxMzU5MzYuNjAuMC4w.
- Agni Nakou, Dragioti, E., Bastas, N.-S., Nektaria Zagorianakou, Varvara Kakaidi, Dimitrios Tsartsalis, Stefanos Mantzoukas, Fotios Tatsis, Veronese, N., Solmi, M. and Gouva, M. (2025). Loneliness, social isolation, and living alone: a comprehensive systematic review, meta-analysis, and meta-regression of mortality risks in older adults. Aging Clinical and Experimental Research, 37(1). doi:https://doi.org/10.1007/s40520-024-02925-1.
- Faculty of Public Health (2024). Faculty of Public Health Briefing: Housing, poverty & public health. [online] Faculty of Public Health. Available at: https://www.fph.org.uk/media/p5rdhsu5/fph-poverty-housing-and-health-briefing.pdf.
- Office for National Statistics (2020). Living longer - Office for National Statistics. [online] www.ons.gov.uk. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/livinglonger/changesinhousingtenureovertime.
- Public Health Wales. (2024). Cancer Reporting Tool - Official Statistics. [online] Available at: https://phw.nhs.wales/services-and-teams/welsh-cancer-intelligence-and-surveillance-unit-wcisu/cancer-reporting-tool-official-statistics/.
- Office For National Statistics (2024). Adult Smoking Habits in the UK: 2023. [online] Ons.gov.uk. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2023.
- Li, S., He, Y., Liu, J., Chen, K., Yang, Y., Tao, K., Yang, J., Luo, K. and Ma, X. (2024). An umbrella review of socioeconomic status and cancer. Nature Communications, [online] 15(1). doi:https://doi.org/10.1038/s41467-024-54444-2.
- Banks, I. (2001). No man’s land: men, illness, and the NHS. BMJ, [online] 323(7320), pp.1058–1060. doi:https://doi.org/10.1136/bmj.323.7320.1058.
- McCutchan, G., Wood, F., Smits, S., Edwards, A. and Brain, K. (2016). Barriers to cancer symptom presentation among people from low socioeconomic groups: a qualitative study. BMC Public Health, 16(1). doi:https://doi.org/10.1186/s12889-016-3733-2.